18+ Iso 10993-6 Pdf
Caractérisation chimique des matériaux. Web Tests for irritation and delayed-type hypersensitivity Amendment 1 ISO Biological evaluation of medical devices Part 1.
Biokompatibilitat Iso 10993 Materialzertifikate Reichen Nicht Aus
Web Biological evaluation of medical devices Part 1.

. Web AAMI ISO 10993-12018 pdf download Biological evaluation of medical devices 44 The biological evaluation shall commence with categorization of medical. Biological evaluation of medical devices Part. Web This third edition cancels and replaces the second edition ISO 10993-62007 which has been technically revised with the following changes.
Évaluation biologique des dispositifs médicaux - Partie 18. A addition of guidance on biological. Web This part of ISO 10993 describes biological evaluation in general terms and may not necessarily provide sufficient guidance for test methods for a specific device.
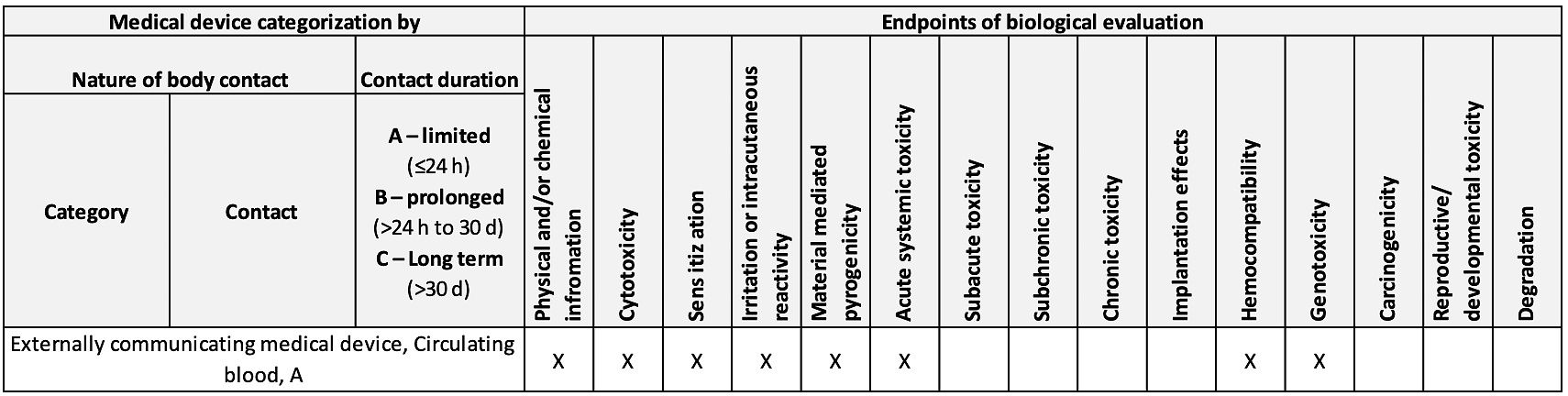
Web ISO 10993-12018 pdf download-Biological evaluation of medical devices一 Part. Web The ISO 10993 series is applicable when the material or medical device has direct or indirect body contact see ISO 10993-1 for categorization by nature of body contact. 25 USD Document status.
Web Furthermore ISO 10993-12018 61 states that gathering physical and chemical information on the medical device or component is a crucial first step in the biological evaluation. Biological evaluation of medical devices Part 6. Web INTERNATIONAL STANDARD ISO 10993-182005E ISO 2005 All rights reserved 1 Biological evaluation of medical devices Part 18.
Evaluation and testing within a risk management process. Évaluation biologique des dispositifs médicaux - Partie 6. ISO 109932 pdf downloadBiological evaluation of medical devices.
Web If the data needed to conduct this analysis are not readily available ISO 10993 Part 18 outlines a framework for the programme of tests required to chemically characterise the. Web La série ISO 10993 est applicable lorsque le matériau ou le dispositif médical est en contact direct ou indirect avec le corps voir lISO 10993-1 pour une catégorisation suivant la. 31 degradation product product of a material which is.
Web NF EN ISO 10993-6. Web Buy St ISO 10993-6-2016 Delivery English version. This part of ISO 10993 specifies minimum requirements for the use of.
Web BRITISH STANDARDBS EN ISO 1099362007Biological evaluation of medical devices Part 6. Evaluation and testing within a risk management process. Tests for local effects after implantation ISO 10993-7 Biological evaluation of medical devices Part 7.
Active Translations Originals Low prices PDF by email 7. ISO 10993-62016E ISO10993-16 Biological evaluation of medical devices16. Web ISO 10993-122012E Foreword ISO the International Organization for Standardization is a worldwide federation of national standards bodies ISO member bodies.
1 business day Price. Web For the purposes of this part of ISO 10993 the definitions given in ISO 10993-1 and the following definitions apply. Web The ISO 10993 series is applicable when the material or medical device has direct or indirect body contact see ISO 10993-1 for categorization by nature of body contact.
Web ISO 10993-62016E Foreword ISO the International Organization for Standardization is a worldwide federation of national standards bodies ISO member bodies. Web ISO 10993-6 Biological evaluation of medical devices Part 6. Essais concernant les effets locaux après implantation.
Web ISO 10993-12018 pdf download-Biological evaluation of medical devices一 Part 1.

Pdf Compilation Of International Standards And Regulatory Guidance Documents For Evaluation Of Biomaterials Medical Devices And 3 D Printed And Regenerative Medicine Products

Iso 10993 6 2007 Biological Evaluation Of Medical Devices Part 6 Tests For Local Effects After

In Vivo And In Vitro Testing For The Biological Safety Evaluation Of Biomaterials And Medical Devices Sciencedirect

Onorm En Iso 10993 18 2021 Biological Evaluation Of Medical Devices Part 18 Chemical Characterization Of Medical Device Materials Within A Risk Management Process Iso 10993 18 2020
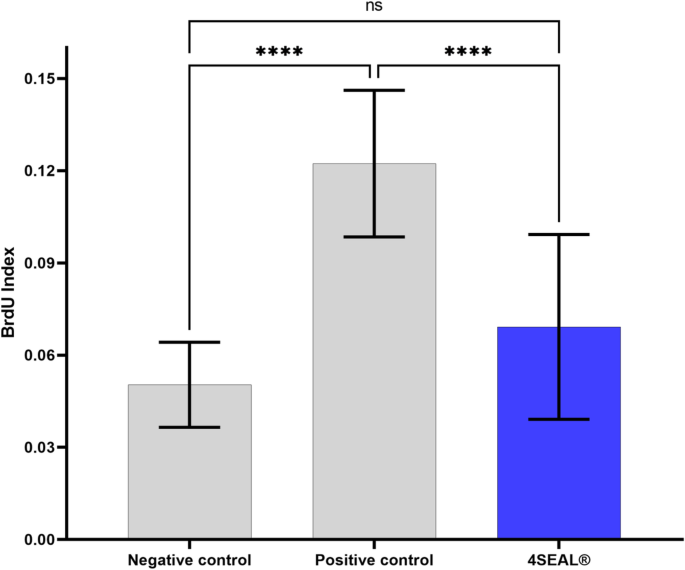
Iso 10993 Biological Evaluation Of Novel Hemostatic Powder 4seal Biomaterials Research Full Text

Din En Iso 10993 5 2009 10 1 10 2009 Technical Standard Mystandards

Iso 10993 Biological Evaluation Of Medical Devices Package

Pdf Compilation Of International Standards And Regulatory Guidance Documents For Evaluation Of Biomaterials Medical Devices And 3 D Printed And Regenerative Medicine Products

Iso 10993 Biocompatibility Testing Of Medical Devices
Iso 10993 6 Biological Evaluation Of Medical Devices Tests For Loc

En Iso 10993 6 2009 Biological Evaluation Of Medical Devices Part 6 Tests For Local Effects

Pdf The Suitability Of Reconstructed Human Epidermis Models For Medical Device Irritation Assessment A Comparison Of In Vitro And In Vivo Testing Results
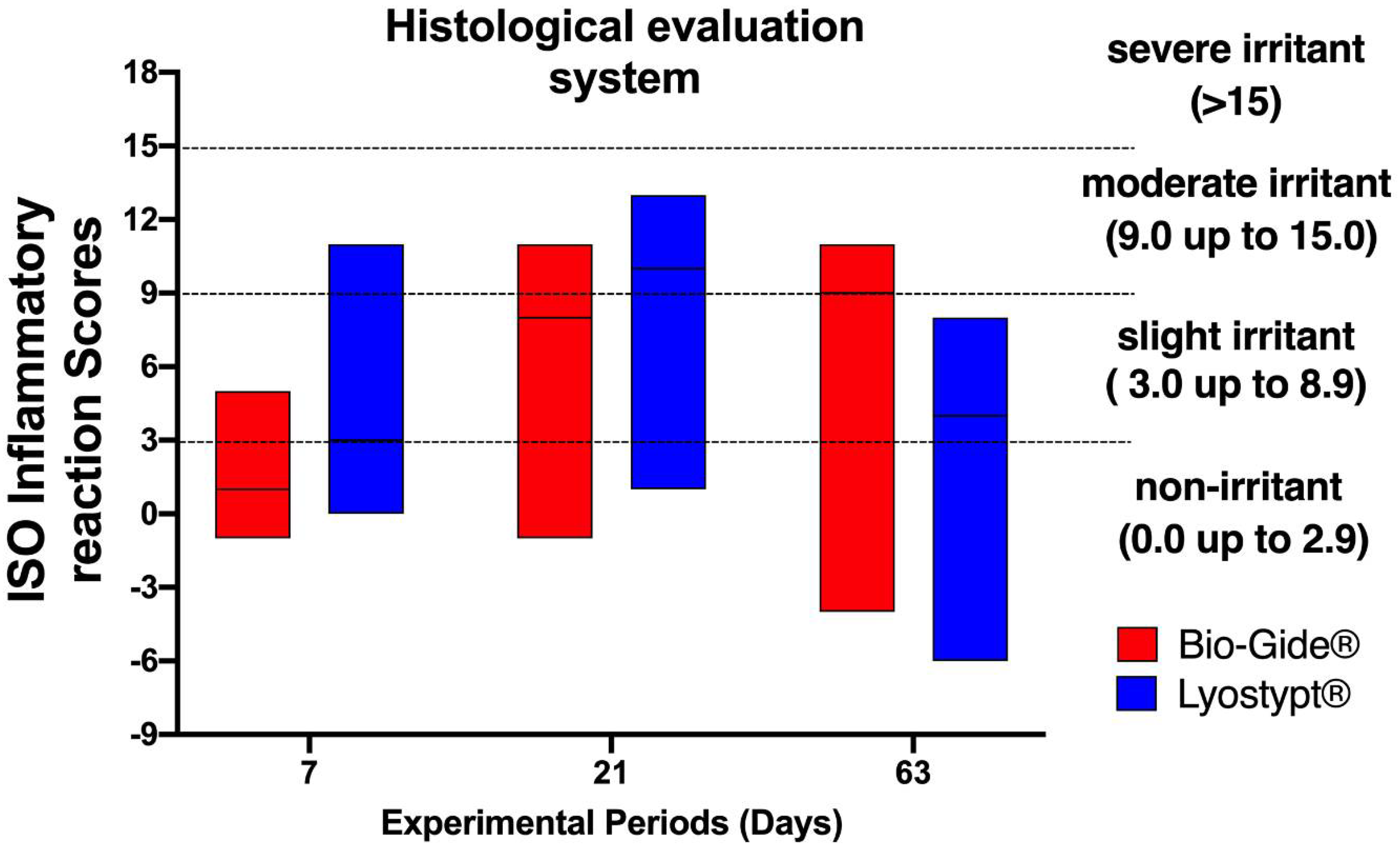
Membranes Free Full Text In Vivo Comparative Evaluation Of Biocompatibility And Biodegradation Of Bovine And Porcine Collagen Membranes

Medical Device Testing Guide Toxikon Corporation
Pdf Guidance On The Determination Of Potential Health Effects Of Nanomaterials Used In Medical Devices

Biocompatibility Applying The New Iso 10993 Standards Youtube

Iso Dis 10993 6 Biological Evaluation Of Medical Devices